Certified Compliance Training for Life Sciences
GxP Training is an accredited online courses catalog designed to assist quality professionals to meet their regulatory requirements
⭐️⭐️⭐️⭐️⭐️ 4.80 based on 4550 reviews


Trusted Certification
100% Online
CPD / CEU Accreditation
+3.000 organizations and individuals have been certified










85+
Online GxP training courses
130+
hours of training
24/7
Anywhere, anytime. Accessible to your team, on your schedule
12+
Training assets updated every month
Main training categories


GDP : Good Distribution Practices

GLP : Good Laboratory Practices

GMP : Good Manufacturing Practices
Medical Devices

Pharmacovigilance

Quality Assurance

Regulatory Affairs

Food and Nutrition
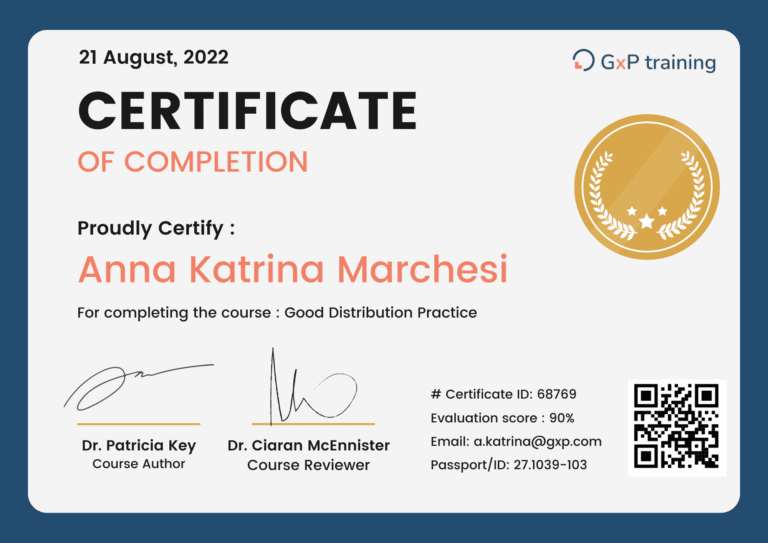
Get your certification
We ensure our learners, customers and other participants to get the best return on their investment by providing a 21 CFR PART 11 compliant certificate when a course is successfully completed.
GxP Training certificates are transferable and recognized by auditors, regulatory boards corporations and universities. Most of our online courses are CEU / CPD accredited.
Now you can take any of our courses and learning paths and receive official certificates each time.