
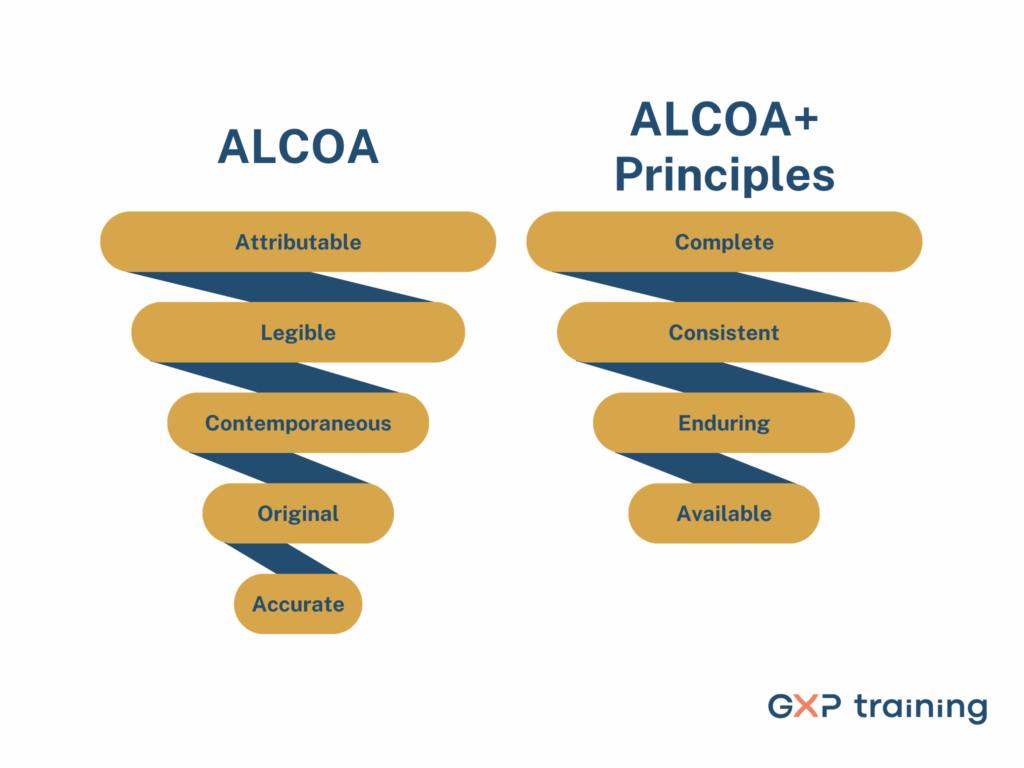
When it comes to quality and compliance in life sciences, few concepts are as foundational as ALCOA+ training requirements. These principles include Attributable, Legible, Contemporaneous, Original, and Accurate, along with Complete, Consistent, Enduring, and Available. They shape the way data should be handled across regulated environments.They guide how information should be recorded, maintained, and accessed throughout the product lifecycle.
But while most professionals working in regulated environments are familiar with the term, the real question is, how often should training on ALCOA+ take place, and who exactly needs it?
In this blog, we’ll unpack what ALCOA+ means in practice, explore whether annual training is a must, and outline which teams should be fully up to speed. If you’re looking to strengthen your data integrity program or reduce compliance risk, this is a great place to start.
ALCOA+ is more than just a list of expectations. It’s about how data should be handled day to day, especially in regulated environments. Each part of it helps make sure the information you rely on is clear, correct, and trustworthy.
Here’s a simple breakdown:
Attributable: It’s always clear who recorded the data or made a change.
Legible: The information is easy to read and won’t be misinterpreted.
Contemporaneous: The data is recorded at the time the task is done, not later.
Original: The record is either the first version or a verified copy.
Accurate: The data reflects what actually happened, without errors.
The “plus” in ALCOA+ adds a few more important ideas:
Complete: Nothing is left out or skipped.
Consistent: The data follows a reliable and expected format.
Enduring: It’s stored in a way that keeps it safe for as long as it’s needed.
Available: You can access it easily whenever it’s needed, whether that’s for daily work, audits, or anything else.
Together, these principles shape how good data management should look. They apply to paper records, electronic systems, and everything in between.
A common question that comes up is whether teams need to do ALCOA+ training every year. The truth is, there’s no official rule that says it must happen annually. However, most regulators expect companies to train staff regularly, especially those who handle regulated data or work in GxP environments.
In practice, many organisations choose to offer ALCOA+ training once a year. This gives teams a chance to refresh their understanding, stay up to date with any changes, and keep good habits in place. It also helps make sure new employees are brought up to speed and that everyone is on the same page when it comes to how data should be handled.
Even though annual training isn’t a hard requirement, it’s widely seen as a smart approach. It supports a culture of accountability and gives teams the knowledge they need to do their jobs well, without cutting corners.
ALCOA+ principles apply to anyone who works with regulated data, which means the list of people who need training can be surprisingly broad. It’s not just for quality teams or lab staff. It includes anyone who records, handles, or reviews data as part of their role.
This often includes lab technicians, quality control and quality assurance staff, and anyone involved in manufacturing, clinical operations, or regulatory affairs. It also includes IT staff who manage systems that store or process data, especially if those systems are used in a GxP setting.
Even administrative teams and contractors may need training if they’re entering or managing records that relate to compliance. The key is to think about data ownership and responsibility. If someone plays a part in how data is created, reviewed, or stored, they should understand what ALCOA+ means and how it affects their work.
Making this training role-specific is important. The goal isn’t to teach everyone the same thing, but to make sure each person understands the parts of ALCOA+ that relate to their job and the systems they use.
When people really understand ALCOA+, they make better decisions in how they record and handle information. Our Pharmaceutical Data Integrity: ALCOA and ALCOA+ course gives teams clear, practical guidance they can actually apply to daily work. It explains what good data integrity looks like, why it matters for inspections, and how to build habits that protect records from start to finish.
This course is built by experienced regulatory professionals who know what auditors look for and how to connect those expectations to real tasks. Staff come away knowing how to spot problems early, handle records correctly, and support the overall quality culture in your organisation.

Every learner gets an accredited certificate that can be verified and shared with confidence, showing that your company takes data integrity seriously.
Key benefits include:
This training helps your team feel prepared, not just compliant. If you want to go further, you can also browse our full GxP course library to strengthen other areas of your quality program.
Not all training sessions leave a lasting impression. To make ALCOA+ training useful, it has to feel relevant and practical. People remember what sticks with their day-to-day work, not just what’s written in a policy.
The most effective training is tailored to the roles people actually have. For someone working in the lab, it might focus on how to document results in real time. For someone in quality assurance, it could cover how to spot incomplete records or inconsistencies. The point is to keep the content grounded in real situations, not just theory.
Interactive formats often work better than lectures or long slideshows. Case studies, real examples, and group discussions help people connect the dots between the principles and the decisions they make every day. It’s also useful to include reminders of how ALCOA+ training requirements link into other areas, like document control, CAPA investigations, or system validation.
Good training is part of onboarding, part of change management, and part of how teams grow. When handled well, it becomes part of the culture.
It’s easy to think of training as something you just have to get through, but when it comes to ALCOA+ training requirements, regular refreshers can make a real difference. These principles aren’t just theoretical, they’re a big part of how companies stay compliant and protect the quality of their work.
When teams understand what’s expected and why it matters, they’re more likely to catch issues early, document things correctly, and avoid mistakes that could lead to audit findings or delays. Over time, this builds confidence and consistency across departments.
Regular training also helps when systems or procedures change. Whether it’s a new piece of software or a shift in how records are reviewed, ALCOA+ gives teams a shared language for handling data the right way, no matter what tools they’re using.
Most importantly, it reinforces the idea that data integrity is everyone’s responsibility. That mindset is what turns training from a one-time task into something that shapes how teams work every day.
Getting ALCOA+ training right helps your team build habits that protect data integrity every day. If you’re ready to make it simple, our certified Pharmaceutical Data Integrity: ALCOA and ALCOA+ course gives clear, practical guidance and an audit-ready certificate.
You can also explore our full GxP training library and talk to us about what will work best for your team.