
Clinical trial teams handle a huge amount of data every day. Whether it’s patient information, lab results, or notes from site visits, every detail needs to be recorded carefully and kept accurate. That’s not always easy, especially when timelines are tight and systems vary from site to site. This is why it’s so important that staff understand clinical trial data integrity risks and how to manage them effectively from the start. When people know what to watch out for, they’re better prepared to catch small issues early and avoid bigger problems later on.
This blog looks at practical ways to help your team understand the risks around data integrity in clinical trials. It’s about creating the right habits from the start so that everyone handles data properly and consistently, every step of the way.
In clinical trials, everything depends on trustworthy data. It is what regulators review, what scientists rely on, and what ultimately shapes patient care. Even small mistakes, like a missed entry or an unauthorised change, can set a study back by weeks or months. In some cases, those mistakes may cast doubt on the results entirely.
This is why your team’s understanding of data integrity risks needs to go beyond the basics. When people know how errors happen and what they look like, they become more careful. They pay closer attention when recording results, follow procedures more closely, and are quicker to speak up if something feels off.
Training is only part of the answer. What really makes a difference is building habits. When data quality becomes part of the team’s day-to-day thinking, it becomes second nature. That kind of mindset is what keeps trials on track and protects the credibility of your results. For a deeper understanding of regulatory expectations, teams can refer to the FDA guidance on data integrity, which outlines common pitfalls and best practices for handling clinical data.
Managing clinical data the right way means giving your team training they can actually use. Our Clinical Data Management course is clear, practical, and built by regulatory experts who understand how daily work really happens. It covers Good Clinical Practice principles, the key phases of clinical trials, and how to handle data from start to finish so your records stand up to scrutiny.
The course is easy to follow and shows real examples of how to keep data reliable. It also helps your staff get comfortable with electronic systems and learn how to protect data accuracy throughout every step.

Everyone who completes the training gets a traceable certificate that proves they know what they’re doing. You can rely on features that make it simple to use and manage:
If your team works with clinical trial data, this course can help them get it right. You can also browse our full GxP training library to see what other topics fit your goals.
Helping your staff truly understand clinical trial data integrity risks takes more than a one-off training session. It means bringing the conversation into everyday work and making sure the message lands in a way that feels relevant and practical.
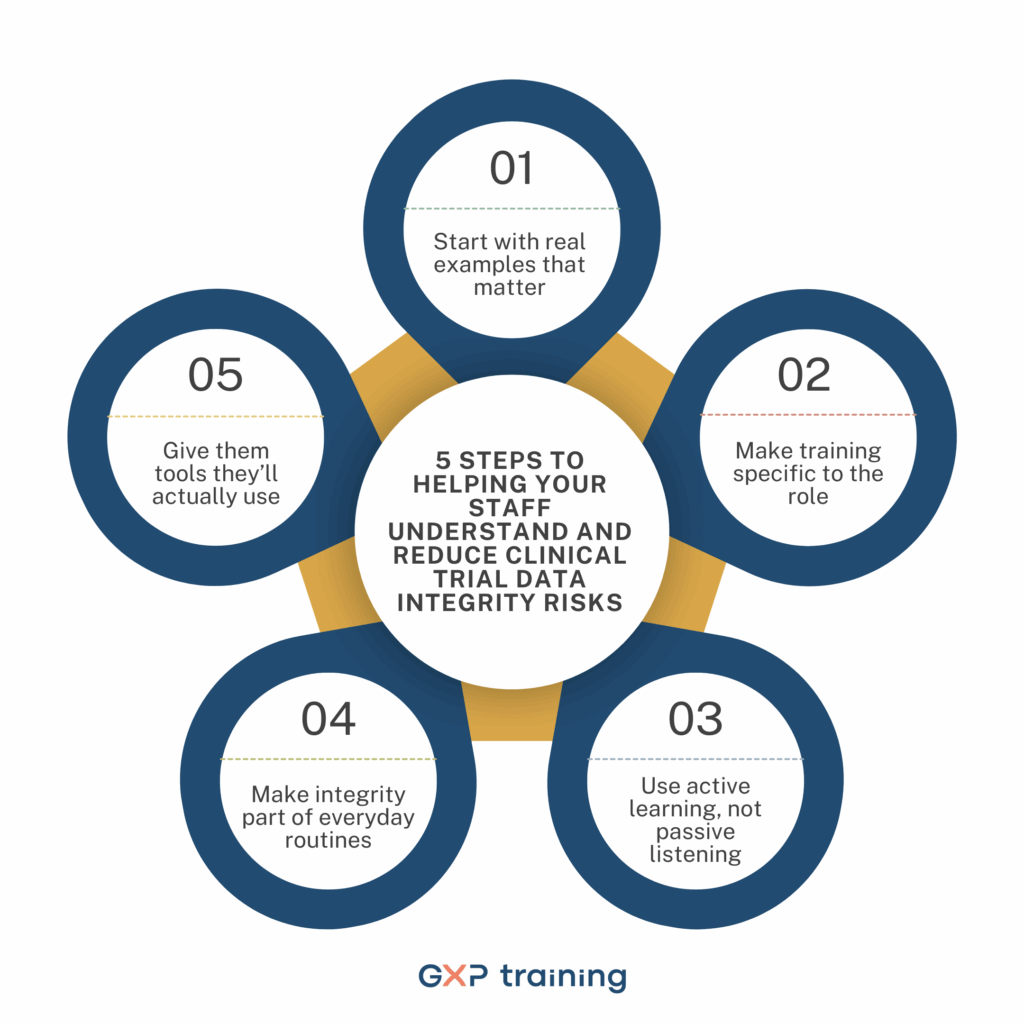
Understanding data integrity risks shouldn’t stop after one training session. It’s something that needs to be revisited regularly so that it becomes second nature rather than something people only think about when things go wrong.
One of the best ways to keep this knowledge fresh is to schedule short refreshers throughout the year. These don’t need to be long or formal, but they should be timely. For example, after a system upgrade or a change in procedures, bring the team together for a quick review of what’s different and why it matters.
Real-life examples are powerful here. Without naming names, you can share stories about recent audit findings or situations where things nearly went off track. These moments are valuable reminders of how small mistakes can lead to bigger problems. When staff see how these risks play out in real scenarios, it becomes easier for them to recognise similar patterns in their own work.
It’s also helpful to review audit logs and monitoring reports regularly. Not just as a compliance check, but as a way to spot patterns. Maybe a specific team keeps forgetting to complete a step, or a recurring error keeps showing up in one system. These insights are opportunities to improve, and they should be shared with the team in a constructive way. Think of it as a conversation, not a criticism.
Keeping the conversation going, even in small ways, helps build a stronger culture. It reminds everyone that protecting data is part of the job, every day, in every role. Over time, this approach creates habits that stick and helps make sure that your trial stays on track.
One of the best ways to protect data integrity is to build a team culture where speaking up is seen as a strength, not a risk. When people feel safe raising questions or pointing out something that doesn’t look quite right, you’re far more likely to catch small problems before they turn into larger issues.
Let your team know that bringing up concerns is not only welcome but appreciated. Whether it’s a technician who notices an unusual timestamp or an administrator unsure about how to log a correction, those small moments of hesitation should be seen as opportunities for improvement, not mistakes.
Create an environment where people feel confident asking questions. This starts with leadership. If managers respond calmly and constructively when something is flagged, others will follow suit. Simple messages like “Thanks for catching that” or “Good call bringing this up” go a long way toward encouraging open dialogue.
It also helps to make it easy for people to report concerns, whether that’s through regular team check-ins, anonymous feedback channels, or a straightforward escalation process. The key is to remove the fear of being judged or getting someone in trouble. When people know they won’t be blamed for asking a question or spotting a potential issue, they’re much more likely to speak up when it counts.
Over time, this mindset becomes part of the everyday rhythm of the workplace. It makes teams more alert, more supportive, and more focused on getting things right, together. And that’s exactly the kind of culture that keeps data safe and trials on track.
Helping your team truly understand clinical trial data integrity risks is what keeps your research credible and your submissions strong. When people know what to look for and feel confident speaking up, small errors are spotted before they grow into real problems.
If you want a simple way to build this confidence, our Clinical Data Management course gives your team practical skills they can apply right away. You can also browse our full GxP training catalogue to find other courses that fit your needs. Take a look and see how we can help keep your trials on track.