
In clinical research, the integrity of your trial depends not only on rigorous protocols and meticulous data collection, but also on the competence of every individual involved. When regulators arrive for a Good Clinical Practice (GCP) inspection, one of their first stops is your training compliance file. This file tells the story of how your team has been prepared to uphold participant safety, data integrity and regulatory standards. A disorganized or incomplete file can turn a routine audit into a cascade of observations, delays and potential enforcement actions. In this blog, we will guide you through crafting an audit-ready training compliance file and show how GxP Training’s Clinical Trials : Preparing for an Audit or an Inspection course equips your team with the knowledge, templates and certificates needed to succeed.
Regulators around the world expect documented proof that everyone on your trial team is qualified by education, training and experience for their assigned responsibilities. The ICH GCP E6(R2) guideline explicitly requires that personnel maintain training records demonstrating competency in their roles. In the United States, FDA’s 21 CFR Part 11 governs electronic records and signatures, meaning your digital training logs must include secure audit trails, time stamps and user authentication. Across the European Union, the EU Clinical Trial Regulation mandates that both sponsor and investigator staff receive training on GCP principles and local regulatory obligations.
During an inspection, auditors will examine three core attributes of your training records: completeness, currency and traceability. Completeness means your file covers every team member from their hire date onward. Currency ensures that refresher training and renewals are documented before certificates expire. Traceability requires that each record clearly links a participant to a specific training module, date, trainer name and signature or electronic confirmation. Version control is all-important. If you updated your Standard Operating Procedures or training slides, you must show that staff completed the correct version at the time of training.
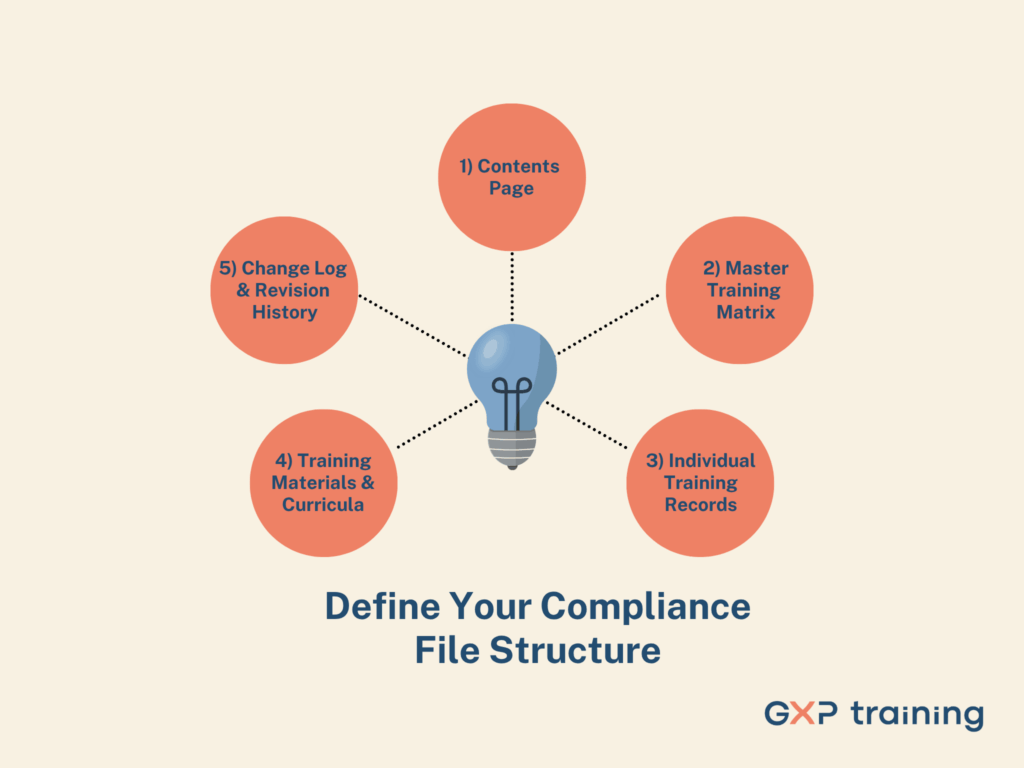
A consistent, logical folder and file naming structure forms the backbone of an audit-ready compliance file. A clear roadmap helps auditors and your team find exactly what they need in seconds.
Your file needs these sections:
This structure creates a transparent, user-friendly file demonstrating control and traceability. Auditors will appreciate the logical organisation and quick access to all necessary records.
A robust training compliance file is about giving your team the confidence and know-how to navigate every step of a GCP inspection. Reading a regulation is one thing, knowing exactly how to prepare for an inspector’s questions, organise your evidence and respond to findings takes hands-on, practical training that mirrors real-world audit scenarios.
Our Clinical Trials : Preparing for an Audit or an Inspection course is designed to do exactly that. This two-hour, interactive online program breaks down the inspection process, from initial document review through on-site audit activities, and shows your team how to anticipate auditor expectations at sponsor, CRO and investigatory sites. Built by Regulatory Affairs experts with Northeastern University qualifications and vetted by senior auditors, the course blends theory with real-life examples and ready-to-use tools.
Every participant completes a short final exam to reinforce critical concepts and earns a downloadable, 21 CFR Part 11-compliant certificate of completion. These CEU/CPD-accredited certificates are shareable on LinkedIn and verifiable via our online certificate checker, giving you traceable proof to include in your training matrix.

What makes this training work:
When your team trains with this course, you’ll have the practical skills, documentation and audit-ready evidence to demonstrate compliance from day one.
Once you understand the regulations and have structured your folders, it’s time to gather the important files. Begin by exporting your master training matrix from your Learning Management System. This export should capture employee identifiers, job roles, required modules, including our audit-preparation course, completion dates and next due dates.
For each individual, file their course certificate alongside any signed attendance logs or assessment reports. If on-the-job training or ad hoc refreshers occur, document these with brief summaries on a standard acknowledgement form signed by the participant and their supervisor. In your training materials section, include the course syllabus, slide decks and any SOP excerpts cited during instruction, making sure each document displays its effective date and version number.
Retain copies of any quizzes or exam results. These not only demonstrate competency thresholds but also serve as evidence that learners understood critical concepts. Organize all these artifacts under employee folders, making sure that an auditor opening a single folder can trace every aspect of that person’s training history.
Traceability extends beyond filing. Your compliance file must show who approved each training event. For every certificate or attendance sheet, include a signature or electronic verification by the trainer or course facilitator and the participant’s direct supervisor or quality lead. If you use electronic signatures, confirm that your system meets all 21 CFR Part 11 requirements, capturing user identity, date and time in an audit trail.
To tie training to actual competence, map each module of the audit-preparation course back to your organization’s role-based competency framework. In your file, include a competency map that shows how lessons on inspection preparation link to specific job descriptions, whether that be a trial manager responsible for oversight or a data coordinator tasked with maintaining audit-ready records.
Before the inspector arrives, conduct an internal self-inspection of your training file. Use a checklist derived from ICH GCP, FDA and EU CTR requirements to verify that no gaps exist in completion dates, signatures or version control. Where you find missing or incomplete records, document the gap and schedule immediate corrective training or file updates. Record these actions in a corrective action log included in your folder.
Presentation matters. In a physical binder, use colour-coded dividers and a quick-start index at the front with key evidence, a snapshot of your master matrix, sample certificates and a list of SOP versions. For digital folders, mirror this structure with well-named subfolders and a hyperlinked contents document that guides auditors to the right files in seconds.
A compliance file is never “finished.” As new hires join your team, schedule their induction training, including Clinical Trials : Preparing for an Audit or an Inspection, within the first month and update your master matrix immediately. When SOPs or regulatory guidelines change, trigger targeted refresher sessions and record them in your change log.
Retention requirements vary by region but typically mandate keeping training records for at least two years after study closure or according to local statutes. Store electronic files in a secure, access-controlled repository and keep any required paper backups in a controlled environment, allowing for rapid retrieval if auditors request archived records.
Preparing an audit-ready training compliance file is a strategic investment in regulatory confidence, product quality and participant safety. When following the structured approach outlined above, and incorporating the expert guidance, templates and verifiable certificates from GxP Training’s Clinical Trials : Preparing for an Audit or an Inspection course, you make sure your team is not only trained but also armed with the evidence auditors seek. Trust GxP Training to help you transform training records from a potential audit liability into a showcase of your organisation’s commitment to clinical excellence.
