Corporate GxP Training
Train and track your team effortlessly with certified, audit-ready courses, all in one seamless platform

Why choosing GxP Training for your organization ?
- HR-ready features to track and manage your team
- 21 CFR PART 11 compliant learning platform
- Training Records Automatically Generated for Regulatory Audits
- Recognized by auditors
- Certified & CPD/CEU Accredited
- SCORM files to import into your in LMS
- Expert-led content, updated at least annually
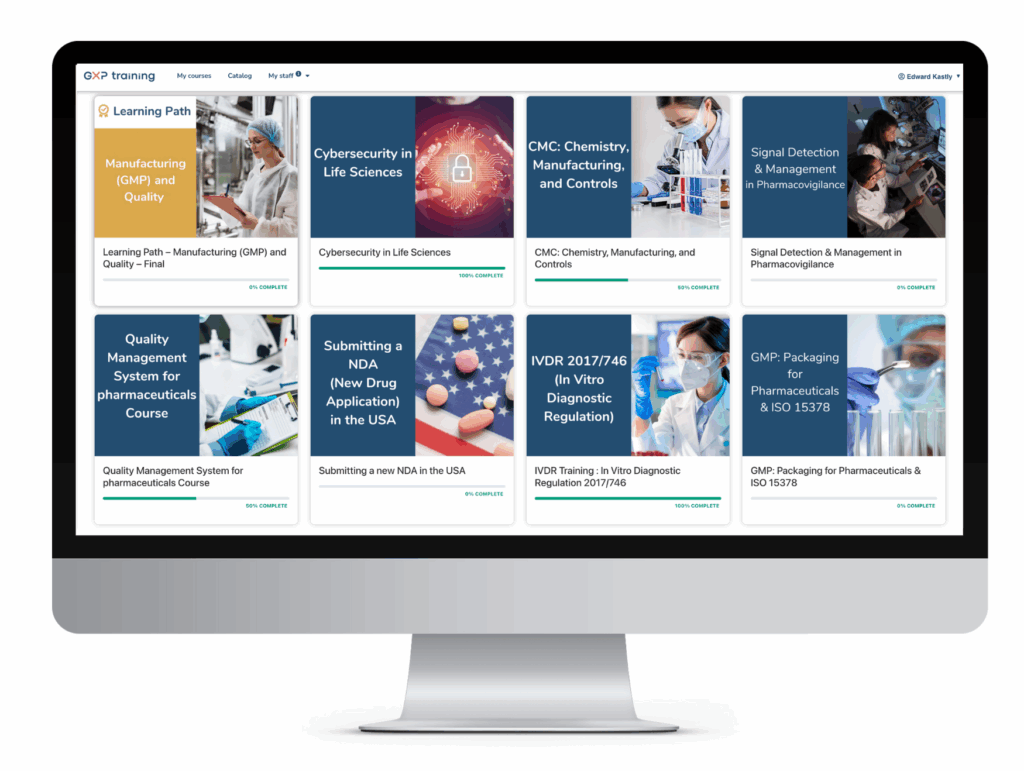
Track the training status of your teams in one place
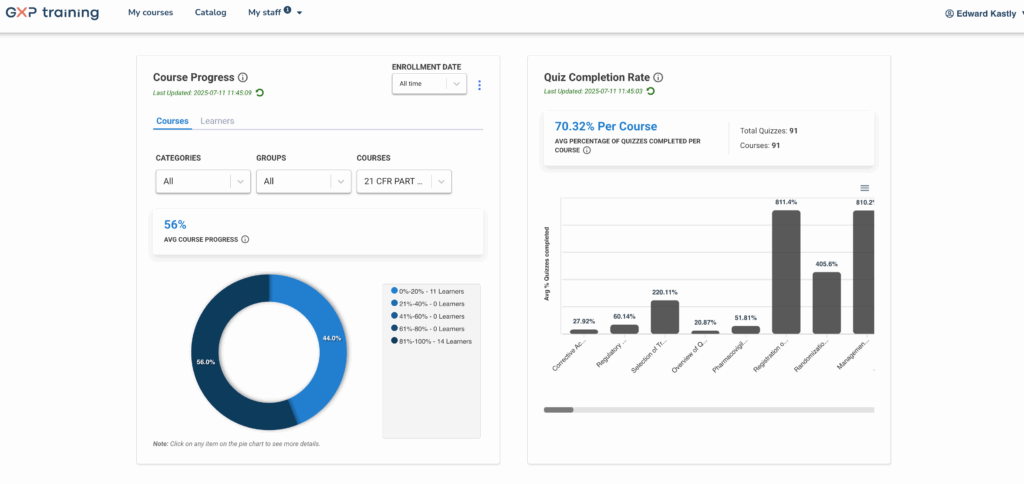
Get detailed statistics by learner, group, course or module:
- Scores
- Certificates
- Learners responses
- Details of the responses
- Feedback analysis
- Time spent
- Engagement and completion rates
Scalable enrollment for teams
Enroll users in purchased courses and organize them into groups based on training requirements. Group leaders can assign training to up to 5,000 learners with a single click.
Generate Training Records
Every training activity is documented, time-stamped, and accessible for inspections or audits. Easily generate audit-ready training records to support compliance and internal reporting.

Training for every phase
From clinical development to post-market activities, our training catalog ensures compliance and consistency across every function in life sciences.
A validated certificate for each learner
When completing a course, we issue an individual completion certificate. Major life sciences companies rely on GxP Training to validate their employees and maintain training records.
All our certificates are unique, authentic, traceable and FDA 21 CFR PART 11 compliant. Each learner will also earn a CPD / CEU credit.

Want to host our courses on your LMS ? We offer SCORM files for easy import into your training platform

